|
Forecast
Period
|
2026-2030
|
|
Market
Size (2024)
|
USD
655.76 Million
|
|
Market
Size (2030)
|
USD
860.90 Million
|
|
CAGR
(2025-2030)
|
4.60%
|
|
Fastest
Growing Segment
|
Small
Molecules
|
|
Largest
Market
|
West
India
|
Market Overview
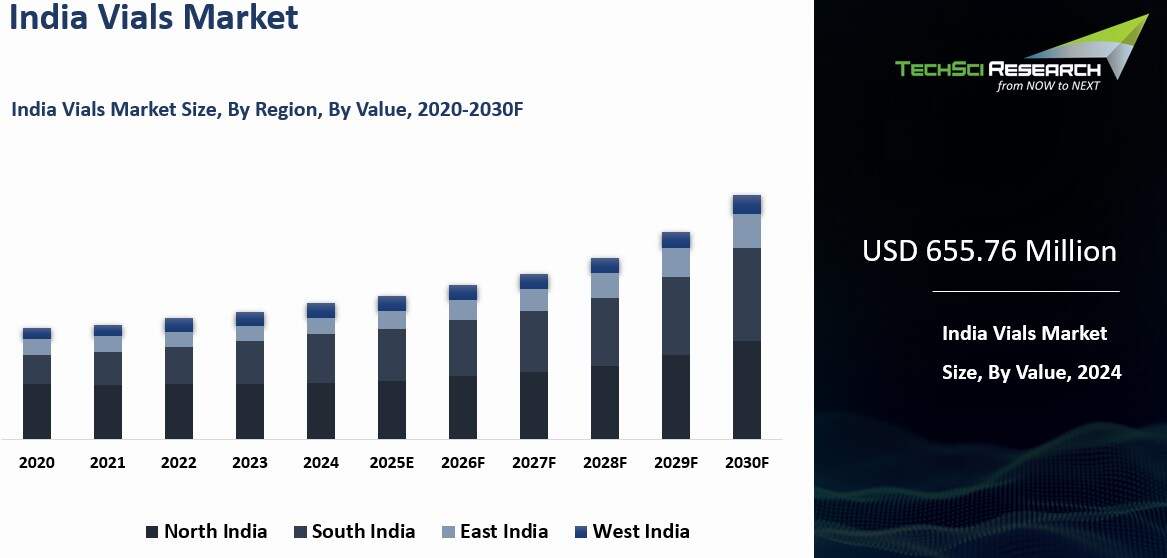
India Vials Market was valued at USD 655.76 Million in 2024 and is expected to grow to USD 860.90 Million by 2030 with a CAGR of 4.60% during the forecast period.
The India vials market plays a critical role in the country’s pharmaceutical
packaging sector, focused on the production and distribution of vials for
storing and administering injectable medications. This market has seen robust
growth, fueled by the increasing demand for injectable therapeutics, the
expanding pharmaceutical industry, and ongoing advancements in packaging
technologies.
Manufacturers are required to comply with stringent regulations,
including the standards outlined by the Central Drugs Standard Control
Organization (CDSCO) and Good Manufacturing Practices (GMP), ensuring the
quality and safety of products. As the demand for injectable drugs rises,
particularly due to the growing prevalence of chronic diseases, and as
innovations in packaging technologies continue to emerge, the market is
positioned for further expansion. To capitalize on this growth, stakeholders
must prioritize strategic investments in production capabilities while
maintaining strict adherence to regulatory frameworks to remain competitive and
ensure compliance.
Key Market Drivers
Expansion of the
Pharmaceutical Industry
The
expansion of the pharmaceutical industry in India is a critical factor driving
the growth of the Indian vials market. As the pharmaceutical sector in India
continues to evolve and expand, the demand for injectable drugs, biologics, and
vaccines has surged, creating a direct need for reliable and efficient
packaging solutions such as vials.
The growing focus on injectable medications,
driven by the need for treatments in chronic diseases such as cancer, diabetes,
and autoimmune disorders, has directly influenced the demand for vials.
Injectable drugs are essential for administering therapeutics that cannot be
delivered orally, such as biologics and vaccines. As the pharmaceutical
industry increases its production of these injectables to meet both domestic
and global demand, the need for vials as essential packaging components also
rises.
This includes vials designed for both single-use and multi-dose
applications, as well as those tailored for specific drug formulations. India's expanding middle class, now comprising 41% of the nation's 1.4 billion population, has led to increased disposable income, making medicines more accessible. Additionally, the growth of health insurance providers has further bolstered this accessibility. Currently, India relies on imports for nearly 70% of its active pharmaceutical ingredient (API) needs, predominantly from China. The government's policy outlines ambitious goals to boost the domestic production of APIs, aiming to significantly strengthen India's pharmaceutical security.
India's
pharmaceutical industry is increasingly focusing on the development of
biopharmaceuticals, which require specialized packaging due to their complexity
and sensitivity. Biologics, such as monoclonal antibodies and gene therapies,
are typically delivered through injections and require highly regulated,
secure, and durable packaging solutions like vials.
The expansion of India’s
biopharmaceutical sector is thus driving the need for specialized vials that
meet stringent regulatory standards and ensure the stability and safety of
these sensitive drugs. As India aims to become a global hub for biosimilars and
other biologics, the demand for vials designed to store and deliver these
treatments continues to rise. India plays a pivotal role in global vaccine
production, with many domestic manufacturers supplying vaccines for both
national and international markets.
The rise in vaccination programs,
especially during the COVID-19 pandemic and in response to other infectious
diseases, has dramatically increased the demand for vials used to store
vaccines. The government’s continued focus on expanding immunization programs
both domestically and internationally creates a persistent demand for vials
capable of storing large volumes of vaccines under specific temperature
conditions. Additionally, the increased production of vaccines for emerging
diseases further fuels this demand, contributing to the growth of the vials
market.
India
is a major global supplier of generic drugs, which are increasingly being
adopted by countries around the world due to their cost-effectiveness. As the
pharmaceutical industry in India continues to grow, particularly in the generic
drug segment, the need for vials for packaging these drugs also rises. Generic
injectable drugs, which often serve as alternatives to branded biologics,
require vials for storage and transportation.
The continued increase in both
domestic and export sales of generics supports the ongoing demand for vials,
driving growth in the packaging segment. India's pharmaceutical exports have
seen significant growth, particularly in markets like the United States,
Europe, and emerging regions. As Indian pharmaceutical manufacturers continue
to expand their presence globally, they must meet stringent international
packaging standards.
This includes the use of high-quality vials that comply
with regulatory requirements such as the U.S. FDA and European Medicines Agency
(EMA) standards. The increasing volume of exports, especially of injectables
and biologics, directly impacts the demand for vials, as they are integral to
the packaging of these products. As Indian companies scale their operations to
meet international demand, the growth of the vials market is closely linked to
the expansion of India’s pharmaceutical export sector.
The shift towards
patient-centric healthcare is another aspect of the expanding pharmaceutical
industry that drives vial demand. With the rise of self-administered injectable
therapies for conditions like diabetes and rheumatoid arthritis, there is a growing
emphasis on packaging solutions that offer convenience, safety, and ease of use
for patients. Pre-filled vials, which allow for single-dose injections, have
become increasingly popular in this regard. This trend reflects a broader shift
in the pharmaceutical industry towards personalized medicine and home
healthcare solutions, both of which increase the demand for specialized vials
designed for consumer use.
Download Free Sample Report
Surge in Demand for Injectable
Drugs
The
surge in demand for injectable drugs is a significant factor driving the growth
of the Indian vials market. As injectable medications become more integral to
the treatment of a variety of medical conditions, including chronic diseases,
infections, and cancers, the need for high-quality, reliable vial packaging
solutions has sharply increased.
The growing incidence of chronic conditions
such as diabetes (In India, an estimated 77 million adults aged 18 and above are affected by type 2 diabetes, with an additional 25 million individuals classified as prediabetic, placing them at a higher risk of developing diabetes in the near future. Notably, over 50% of those with diabetes remain unaware of their condition, which can lead to severe health complications if left undiagnosed and untreated), cardiovascular diseases, cancer, and autoimmune disorders has
dramatically increased the need for injectable medications.
Injectable drugs,
including insulin, monoclonal antibodies, and biologics, are essential for
treating these chronic conditions. For example, diabetes management
increasingly relies on injectable insulin and other advanced therapeutics,
which must be stored and administered in a secure, sterile manner, often in
vials.
As India experiences a rise in the burden of chronic diseases, the
demand for injectable drugs escalates, thereby driving the corresponding need
for vials. India is estimated to have around 1.1 million people who inject drugs (PWIDs), with an HIV prevalence rate of approximately 10% among this group. While HIV remains a key focus in discussions about injecting drug users (IDUs), there are several other critical health risks to consider. These include various cardiovascular and metabolic factors that pose significant threats to their overall well-being.
Biologics,
including monoclonal antibodies, vaccines, and gene therapies, have
revolutionized the treatment landscape for a variety of diseases, offering
targeted treatments with high efficacy. Biologic drugs are often administered
through injectables and require packaging solutions that ensure product
integrity and sterility. India’s increasing focus on the development and
manufacturing of biologics and biosimilars (biologic medicines that are similar
to innovator drugs) further drives the demand for vials. As these drugs grow in
popularity due to their precision in treatment, vials, particularly those
designed for biologic injectables, become essential components in their
distribution.
India
is a key player in the global vaccine market, both as a producer and
distributor. The increasing global demand for vaccines, particularly in the
wake of the COVID-19 pandemic and growing concerns over infectious diseases,
has led to a surge in vaccine production. Vaccines, which are primarily
delivered via injection, require vials for storage and distribution. With the
expansion of immunization programs both domestically and internationally the
need for vials used in the packaging of vaccines has sharply risen. India’s
extensive vaccine production capabilities, particularly for routine
immunization schedules and emergency health crises, rely heavily on the
availability of high-quality vials.
Biopharmaceuticals, which encompass a wide
range of therapeutic proteins, monoclonal antibodies, and gene therapies, are
increasingly being used to treat conditions that were once considered difficult
to manage. These drugs are typically administered by injection, either in a
clinical setting or at home. The surge in demand for these therapies,
particularly in areas such as oncology, immunology, and rare diseases, directly
impacts the vials market.
The complex nature of biopharmaceuticals, combined
with the sensitivity of these drugs to temperature and environmental
conditions, makes the need for specialized vial packaging solutions even more
critical. As India continues to expand its biopharmaceutical production, the
demand for vials tailored to the specific requirements of these medications
grows. As reported in the 2022 India BioEconomy Report, the biopharmaceutical sector is the largest contributor to India's biotechnology industry, generating an economic impact of approximately USD 39.4 billion in 2021.
The
growing focus on patient-centric healthcare solutions, such as home-based
healthcare and self-administration of medications, has boosted the demand for
injectable drugs. Pre-filled syringes and vials are becoming increasingly
popular for self-injection, allowing patients to manage chronic conditions such
as diabetes, rheumatoid arthritis, and multiple sclerosis from the comfort of
their homes.
The convenience of these solutions has led to higher patient
compliance, which, in turn, drives the market for vials, as they are the key
packaging solution for pre-filled injectables. As patient demand for
easy-to-use, ready-to-administer injectable drugs rises, the vials market is
seeing significant growth.
Government policies and regulatory support aimed at
accelerating the development and manufacturing of injectable drugs further
contribute to the demand for vials. Regulatory bodies such as the Central Drugs
Standard Control Organization (CDSCO) in India are establishing clear
guidelines to promote the production of high-quality injectables.
These
policies facilitate the growth of the injectable drug market by ensuring that
manufacturers meet the necessary standards, which often include requirements
for secure and compliant packaging solutions, such as vials. In response to
these regulations, manufacturers are increasingly investing in advanced vial
technologies to meet the growing demand for injectable drugs.
Increase in Vaccination
Programs and Biologics
The increase in vaccination programs and the expansion of biologics are key factors driving the growth of the Indian vials market. As global and domestic
healthcare priorities shift towards large-scale immunization and the
development of advanced biologic therapies, the demand for vials, which serve
as essential packaging for vaccines and biologics, has surged. This demand is
underpinned by several critical dynamics in the pharmaceutical and healthcare
sectors, all contributing to the acceleration of vial consumption in India. India
plays a pivotal role in global vaccination efforts, both in terms of production
and distribution.
The country is one of the world's largest manufacturers of
vaccines, supplying vaccines for various diseases such as polio, tuberculosis,
hepatitis, and, more recently, COVID-19. The government's ongoing efforts to
expand vaccination programs, aimed at achieving herd immunity and controlling
infectious diseases, have significantly boosted the demand for vials. National
immunization initiatives, such as routine childhood vaccination schedules, and
international campaigns, especially those focused on low- and middle-income
countries, rely heavily on large-scale vaccine production.
Vaccines are
predominantly stored and distributed in vials. With the growing scope of vaccination programs, the need for vials, especially multi-dose vials and those capable of maintaining the integrity of sensitive formulations, has dramatically increased. Furthermore, India's active involvement in global vaccination
efforts, particularly in the context of COVID-19 and other emerging diseases,
has led to increased production volumes. This surge in vaccine production
drives the need for more vials for storage, distribution, and safe
administration. The increase in vaccine production, both domestically and for
export, significantly influences the demand for vials in the market. As of March 4, 2023, India has successfully administered over 2.2 billion doses, encompassing the first, second, and precautionary (booster) doses of the currently approved vaccines.
Biologics
are a rapidly growing segment of the pharmaceutical industry, including
monoclonal antibodies, gene therapies, recombinant proteins, and vaccines.
These drugs are primarily delivered via injectables and require specific
packaging solutions to preserve their potency and stability. The rise of
biologics, which offer targeted treatments for diseases such as cancer,
autoimmune disorders, and genetic conditions, is directly contributing to the
growth of the vials market.
Biologics are typically more complex to manufacture
and store than traditional small-molecule drugs. Their sensitivity to
temperature, light, and handling means that specialized vials are required to
ensure their efficacy and safety throughout their shelf life. As the demand for
biologic drugs in India increases, driven by both domestic healthcare needs and
global exports, the corresponding demand for vials that meet the stringent
requirements for biologics packaging has surged. This includes vials designed
for specific formulations, such as pre-filled syringes and glass vials that
minimize the risk of contamination.
India is a leading supplier of vaccines
worldwide, especially in emerging markets. The global vaccine market has seen
substantial growth, driven by an increasing need for immunization against both
common and emerging diseases. For example, India has been a significant
producer and supplier of COVID-19 vaccines, which are stored and distributed in
vials.
As global vaccination programs expand, particularly in developing
countries, the need for high-quality vials to safely store and transport
vaccines grows. India’s role as a vaccine manufacturing hub has positioned it
as a critical player in global vaccination campaigns. The ongoing international
demand for vaccines, both for routine immunization and emergency health crises,
has spurred the demand for vials.
The country's capacity to produce vaccines on
a large scale necessitates an increased supply of vials, as vaccines are
distributed in large quantities across regions with varying climates and storage
conditions. The Immunization Program is a critical initiative aimed at protecting children from preventable, life-threatening conditions. As one of the largest immunization programs globally, it represents a significant public health effort in the country. The program targets the vaccination of 26 million newborns and 30 million pregnant women each year under the Universal Immunization Program (UIP). Over 9 million immunization sessions are held annually, supported by nearly 27,000 cold chain points nationwide.
With
the development of new vaccines and biologics, including mRNA-based vaccines,
there has been an increasing emphasis on packaging solutions that can meet the
advanced storage and distribution needs of these cutting-edge therapies. The
rise of biologics, particularly vaccines like the mRNA COVID-19 vaccines, which
have specific temperature requirements (e.g., ultra-low temperatures), has led
to the development of specialized vials capable of maintaining these
conditions. As more vaccines and biologic therapies are introduced to the
market, the demand for vials that are compatible with complex storage needs and
high-value drugs continues to rise. Moreover, the trend towards pre-filled
vials and syringes, which are designed to minimize contamination risks and
enhance patient compliance, is becoming more prevalent.
These innovations are
particularly important for biologics, as they require secure, efficient, and
user-friendly packaging. The demand for vials that support these innovations is
helping drive growth in the vials market. Government policies, both within
India and internationally, have played a pivotal role in boosting the
production and distribution of vaccines and biologics. The regulatory
frameworks that support the manufacturing of vaccines and biologics,
particularly in India, are designed to ensure the safety, efficacy, and quality
of these products. These regulations require high standards for packaging,
including vials, to ensure that vaccines and biologics remain stable and secure
throughout their lifecycle.
The regulatory emphasis on Good Manufacturing
Practices (GMP) and the guidelines set forth by bodies such as the Central
Drugs Standard Control Organization (CDSCO) in India, as well as international
regulatory authorities like the World Health Organization (WHO) and the U.S.
FDA, have driven the demand for vials that meet rigorous quality standards.
Manufacturers must ensure that their vials comply with these regulations, which
has spurred innovation in vial design and production, further boosting market growth.
Key Market Challenges
Stringent Regulatory
Compliance and Quality Standards
The
Indian vials market operates in a highly regulated environment, where
manufacturers must comply with rigorous standards and quality controls to
ensure the safety, efficacy, and integrity of pharmaceutical products.
Regulatory bodies such as the Central Drugs Standard Control Organization
(CDSCO) impose strict requirements on packaging materials and processes,
including vials used for injectable drugs, vaccines, and biologics.
Manufacturers
face substantial challenges in meeting both national and international
regulatory standards, which often include stringent testing protocols,
sterilization processes, and packaging materials that can withstand temperature
fluctuations and other environmental factors. Ensuring compliance with these
regulations demands significant investments in quality control, research and
development (R&D), and technology upgrades. For small and medium-sized vial
manufacturers, the cost and complexity of adhering to these standards can be
prohibitive, hindering their ability to scale operations.
Moreover, compliance
with international standards like those set by the U.S. FDA or European
Medicines Agency (EMA) is often necessary for manufacturers targeting global
markets, adding another layer of complexity and cost. As India’s pharmaceutical
exports grow, the pressure to meet these international standards becomes even
more pronounced, limiting the growth potential for some domestic vial
manufacturers. The ongoing need for continuous regulatory updates and
compliance can slow down market agility, presenting a barrier to market
expansion.
Raw Material Shortages and
Price Volatility
The
production of vials, especially glass vials, is highly dependent on the
availability of raw materials such as borosilicate glass, rubber stoppers, and
aluminum seals. India’s vials market is heavily influenced by the fluctuations
in the prices and availability of these critical materials, many of which are
imported. Disruptions in the global supply chain, such as those caused by
geopolitical tensions, trade restrictions, or natural disasters, can lead to
raw material shortages, price hikes, and delays in production.
For
instance, borosilicate glass, which is the preferred material for
pharmaceutical vials due to its resistance to chemical reactions and heat, is
in limited supply and subject to significant price volatility. Similarly, the
rubber and aluminum components that form the closure systems of vials also face
price instability due to global supply chain issues. These fluctuations in raw
material prices impact the overall cost structure of vial manufacturing, making
it difficult for manufacturers to maintain competitive pricing while ensuring
quality. Smaller manufacturers with limited resources may struggle to absorb
these costs, leading to reduced margins or even an inability to meet the
growing demand for vials. In turn, this can restrict the ability of the Indian
vials market to scale effectively, particularly as global demand for vaccines
and biologics increases.
Key Market Trends
Growing Demand for Biologics
and Specialty Drugs
One
of the most significant trends driving the future growth of the India vials
market is the rising demand for biologics and specialty drugs. Biologics,
including monoclonal antibodies, gene therapies, recombinant proteins, and
vaccines, are increasingly becoming the treatment of choice for a wide range of
chronic and complex diseases, such as cancer, autoimmune disorders, and rare
genetic conditions. These biologics are predominantly administered via
injectable formulations, necessitating high-quality vials for storage,
distribution, and administration.
India
has established itself as a major hub for the production of biologics and
biosimilars, with significant investments in biotechnology and
biopharmaceuticals. As the global market for biologics continues to grow,
India’s role in supplying both domestic and international markets is expanding.
The demand for vials, especially for biologics, is intensifying, driven by the
need for specialized packaging solutions that can ensure product integrity,
sterility, and ease of administration. Vials, particularly those designed for
biologics, must meet stringent requirements, such as temperature stability,
tamper-evident closures, and compatibility with self-injection systems. This
trend toward biologics and specialty drugs is expected to be a primary driver
for the growth of the vials market in India.
Technological Advancements in
Packaging Solutions
The
increasing sophistication of injectable drug delivery systems is another key
trend that will significantly impact the future of the India vials market.
Advances in vial packaging technology are leading to the development of
smarter, more user-friendly, and more secure vial solutions. These innovations
include pre-filled syringes, auto-injectors, and multi-dose vials that offer
convenience, reduce the risk of contamination, and improve patient compliance.
Additionally,
the growing adoption of anti-counterfeiting technologies in packaging is
reshaping the vials market. Vials are now being equipped with tamper-evident
seals, RFID tags, and other security features to protect against counterfeit
drugs, which are a growing concern globally. The integration of digital
technologies into vials, such as smart sensors that monitor temperature,
humidity, or even the vial’s usage, is gaining traction. These "smart
vials" offer real-time data, ensuring that drugs are stored and
transported within the recommended conditions, which is critical for biologics
and vaccines. As manufacturers strive to meet the needs of the evolving
pharmaceutical industry, the demand for technologically advanced vials will
increase, thereby driving market growth.
Segmental Insights
Application Insights
Based
on the category of Application, the Small Molecules segment emerged as the
dominant in the India Vials Market in 2024. The small molecules segment has
historically been the largest contributor to the India vials market, accounting
for a significant share of the overall demand for vials. Small molecules,
typically made up of low molecular weight compounds, are used in a wide range
of therapeutic areas, including antibiotics, pain management, cardiovascular
diseases, and other chronic conditions. These drugs are primarily delivered via
oral tablets and injectables, with injectable formulations requiring vials for
storage and administration.
India's
pharmaceutical sector, which is one of the largest in the world, is heavily
focused on the production and export of generic small-molecule drugs. The
country is a global leader in the production of affordable generic medications,
which are primarily injectable forms, driving a substantial demand for vials in
the domestic market. The small molecule market also benefits from its
relatively stable growth trajectory, as these drugs remain the standard
treatment option for many diseases. This has led to consistent demand for
vials, with manufacturers scaling production to meet both domestic consumption
and export needs. These factors are expected to drive the growth of this
segment.

Download Free Sample Report
Regional Insights
West
India emerged as the dominant in the India Vials Market in 2024, holding the
largest market share in terms of value. West India, particularly Maharashtra,
Gujarat, and Goa, is the dominant region in the Indian vials market. This
region is a key hub for the country’s pharmaceutical industry, which has a
direct impact on the demand for vials used in drug storage, packaging, and
administration.
West
India is home to many pharmaceutical manufacturers, including both
multinational corporations (MNCs) and domestic companies. Cities like Mumbai
(Maharashtra) and Ahmedabad (Gujarat) are well-established as centers for
pharmaceutical production, including the manufacturing of injectable drugs,
vaccines, and biologics—all of which require vials for packaging. The presence
of key pharmaceutical players in these regions drives high demand for vials,
particularly for small molecules, biologics, and vaccines. West India also
plays a pivotal role in India’s pharmaceutical export sector.
The region is
strategically located near major ports, such as Mumbai Port and Mundra Port,
facilitating the export of pharmaceutical products and vials to international
markets. As a result, the demand for vials in the region is not only driven by
domestic consumption but also by the need to cater to global markets. West
India boasts a well-developed infrastructure that supports both pharmaceutical
manufacturing and packaging. The region’s advanced research and development
(R&D) facilities, as well as its focus on technological innovations in
packaging, also contribute to the rising demand for vials with specialized
features, such as pre-filled syringes and multi-dose vials.
Recent Developments
- In October 2024, In response to the rising incidents of counterfeit medicines, particularly in the cancer treatment sector, anti-cancer drug vials and strips may soon be mandated to include QR codes and track-and-trace systems. This move aims to enhance the traceability and authenticity of these critical medications, helping to combat the growing threat of fake drugs in the market. The introduction of such technology is expected to bolster the security of drug distribution, ensuring that patients receive legitimate treatments while enabling regulatory bodies to monitor the supply chain more effectively.
- In March 2024, Syngene International, a global contract research, development, and manufacturing organization (CRDMO), announced the operational launch of its newly upgraded biologics facility (Unit 3) in Bangalore, India. The facility was set to support clinical and commercial supply for U.S. and European clients in the latter half of 2024. The facility’s drug substance capacity included two production suites, each equipped with five 2KL single-use bioreactors, totaling a capacity of 20KL. Additionally, the facility featured two high-speed vial filling lines, capable of producing up to one million vials per day, with fill volumes ranging from one to 100mL. Beyond production capacity, the site housed a development suite for the clinical supply of drug substances, which included a 500L single-use bioreactor. Syngene officials stated that further expansion plans were in place, including the addition of two vial filling isolator lines with capacities of 600 vials/minute and 100 vials/minute, respectively, as well as an expansion into perfusion cell culture processing for drug substance production.
- In
June 2023- Corning and SGD Pharma have announced a strategic joint venture to
establish a new glass tubing facility in Telangana, India, aimed at expanding
access to Corning’s Velocity Vial technology. This collaboration combines SGD
Pharma’s expertise in vial conversion with Corning’s advanced glass-coating
technology, marking a significant step toward improving vial quality, enhancing
filling-line productivity, and accelerating the global distribution of
injectable treatments. By breaking ground on this new manufacturing facility,
the two companies are positioning themselves to address the growing
complexities of capacity and quality demands in the pharmaceutical sector,
while supporting the global supply of essential medicines. This partnership is
designed to empower drugmakers with the advanced solutions necessary to meet
the evolving challenges in drug production and delivery.
Key Market Players
- Amcor
Group
- Corning
Incorporated
- Gerresheimer
AG
- KISHORE
GROUP
- Nipro
Medical India Pvt. Ltd
- SCHOTT
Glass India Pvt. Ltd.
- SGD
Pharma
- MITSUBISHI
GAS CHEMICAL COMPANY, INC
|
By
Preparation
|
By
Application
|
By
Material
|
By
End User
|
By
Region
|
- Ready
to use (RTU)
- Ready
to sterilize (RTS)
|
- Small
molecules
- Biologics
- Diagnostics
|
|
- Hospitals
& clinics
- Pharma
& biotech companies
- Contract
development and manufacturing organizations (CDMOs)
- Diagnostic
laboratories
- Other
|
- North
India
- South
India
- West
India
- East
India
|
Report Scope:
In this report, the India Vials Market has been
segmented into the following categories, in addition to the industry trends
which have also been detailed below:
- India Vials Market, By Preparation:
o Ready to use (RTU)
o Ready to sterilize (RTS)
- India Vials Market, By Application:
o Small molecules
o Biologics
o Diagnostics
- India Vials Market, By Material:
o Glass
o Polymer
o Hybrid
- India Vials Market, By End User:
o Hospitals & clinics
o Pharma & biotech companies
o Contract development and manufacturing
organizations (CDMOs)
o Diagnostic laboratories
o Other
- India Vials Market, By Region:
o North India
o South India
o East India
o West India
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the India Vials
Market.
Available Customizations:
India Vials
market report with the given market data, Tech Sci Research offers
customizations according to a company's specific needs. The following
customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional
market players (up to five).
India Vials Market is an upcoming report to be
released soon. If you wish an early delivery of this report or want to confirm
the date of release, please contact us at [email protected]